COMPANY INSIGHT
Sponsored by Cytiva
Implementing the ballroom concept for biomanufacturing: Lessons, reflections and considerations from experience
Picture a large space with elegant couples waltzing around under crystal chandeliers to the sounds of a live ensemble. Now replace the chandeliers with standard industrial lighting, the musicians with arrays of bioprocessing equipment and the dancers with gowned personnel, quietly monitoring and managing the production in an expansive open environment. Welcome to ballroom bioprocessing.

The ballroom concept was originally defined in the International Society of Pharmaceutical Engineers (ISPE) Baseline Guide: Volume 6 – Biopharmaceutical Manufacturing Facilities (2013) as “A large manufacturing area that has no fixed equipment and minimal segregation due to the use of functionally closed systems.”
This concept includes placing the bioprocess equipment on wheels, rather than in permanently fixed positions. Advances in single-use systems and bioprocess automation have facilitated the practicality of closing systems, but the gating factor for industry acceptance was the Pharmaceutical Inspection Co-operation Scheme (PIC/S) determination that clean-room requirements for bioprocessing can be relaxed— or even eliminated—if your process is closed. This signal of acceptance paved the way for industry adoption, which in turn has created new opportunities for risk reduction, cost savings, and facility flexibility.
Early adopters of the ballroom approach have received a lot of attention. Now their ballroom facilities have been inspected and licensed, they have added credibility to the operational feasibility and regulatory acceptance of open architecture suites. But this was often perceived as an approach best suited to large, established biomanufacturers. Questions remained around the practicality around its acceptance in a CDMO facility.
Modified ballroom, dance floor
As originally conceived, the ballroom concept would provide flexibility to deploy equipment to meet the needs of a specific process, scale-up or scale-out by simply wheeling in and connecting additional operations units, or running multiple processes in one space.
Another view of flexibility was quickly revealed in relation to facility design; the ballroom doesn’t have to be a single, large space. It can be distributed over adjacent smaller spaces, connected with through-the-wall connections to maintain process closure. This modified ballroom application, which has come to be known by some as the “dance floor” concept, enables application of the ballroom concept in existing facilities with minimal modification.
Fujifilm Diosynth Biotechnologies had considered a green-field implementation for a new CDMO expansion, but ultimately decided to convert an existing, unused, facility into a modified ballroom design, utilizing single-use equipment. The facility, originally planned as a traditional stainless-steel manufacturing site, was already divided into rooms. Rather than reconfigure the space, it made minor changes and through-the-wall connections to implement a modified ballroom in the existing space.
While most of the manufacturing area does not follow cleanroom requirements, Fujifilm Diosynth decided to implement clean-room standards in the final, post-nanofiltration space. Product at this point is most concentrated, therefore most precious, as it is not remediable should it be contaminated. With the dancefloor implementation, Fujifilm Diosynth could create a clean room in this single area, while avoiding the infrastructure and ongoing costs needed to create a clean-room environment for the entire process. This safeguard also instilled confidence for both the Fujifilm Diosynth staff and their multiple CMO clients.
Maximize flexibility while driving risk out of the system
Fujifilm Diosynth adapted an existing facility, leveraging a ballroom concept to maximize space utilization while minimizing the capital expenditure and the ongoing expenses associated with creating and maintaining a clean-room environment for the entire bioprocess. As a CDMO, it also gained a new level of flexibility to address client needs. But how would clients respond?
The response has been positive, with valuable—and unexpected—lessons learned. Thomas Page, of Fujifilm Diosynth, says “Customers like the robustness of the approach, which goes beyond quality to decrease business and schedule risk. Deploying a closed system in a facility with high level of controls will give a high level of quality, and therefore supports the ability to deliver on time and the ballroom approach provides enhanced risk-control.”
Page believes that closed systems will become an expectation from regulatory agencies. Cytiva’s Parrish Galliher anticipates that bioprocess manufacturers will adopt a similar modified ballroom model, where the seed train and post-filtration operations are in clean rooms, while the upstream, downstream and filtration operations are in a shared space. This environment would have minimal environmental controls but strong closed-system security.
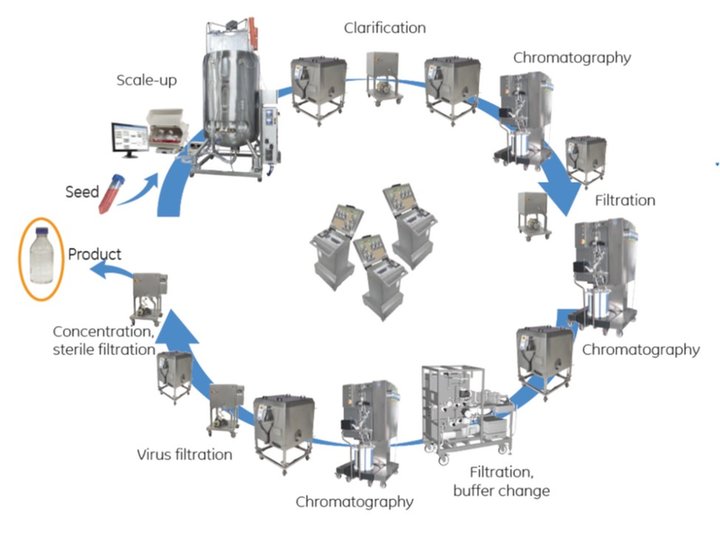
Practical considerations for implementing the ballroom approach
The closed-system dancefloor approach enables biomanufacturers to take full advantage of the flexibility offered by single-use systems by simplifying the logistics, eliminating the need for gowning and the complexity of bringing single-use equipment into a clean-room environment. Parallel process trains can be set up for a single product, or for multiple products, with physical separation of closed systems in a shared space. Robust centralized automation provides the controls needed to manage each process, with segregated products and materials.
After implementing the ballroom and dance floor approach at multiple locations, Galliher has practical advice for biomanufacturers considering this approach.
“Meet with regional regulators early on, still in the design phase. Flag any potential regulatory issues to avoid surprises late in the process. An engineering firm that understands ballroom bioprocessing can help avoid the over-engineering trap and help you realize the full savings of implementing a ballroom for bioprocessing.”
Contact information
Cytiva
Global Life Sciences Solutions USA LLC
100 Results Way,
Marlborough, MA 01752
United States of America