Image: Atomwise CEO and co-founder Abraham Heifets
Osteoarthritis pipeline shifting from analgesics to disease-modifying treatments
Conventional analgesics prescribed for osteoarthritis offer inadequate pain relief and are associated with several adverse events.
Dr Judith M. Sills. Credit: Arriello
Dr Eric Caugant. Credit: Arriello
The current osteoarthritis (OA) market suffers from a lack of effective analgesics and disease-modifying osteoarthritis drugs (DMOADs). These unmet medical needs are reflected in the current OA pipeline, with drugs under development focusing on a variety of novel pain-signalling targets and disease-modifying mechanisms.
OA is a slowly progressive joint disease characterised by degradation of the articular (hyaline) cartilage that coats the bone proximities of synovial joints. Damage to these structures causes irritation and pain, which intensifies with disease progression and can significantly decrease individuals’ quality of life. The sites mainly affected are the weight-bearing joints such as knees, hips, spine and feet, as well as the hands.
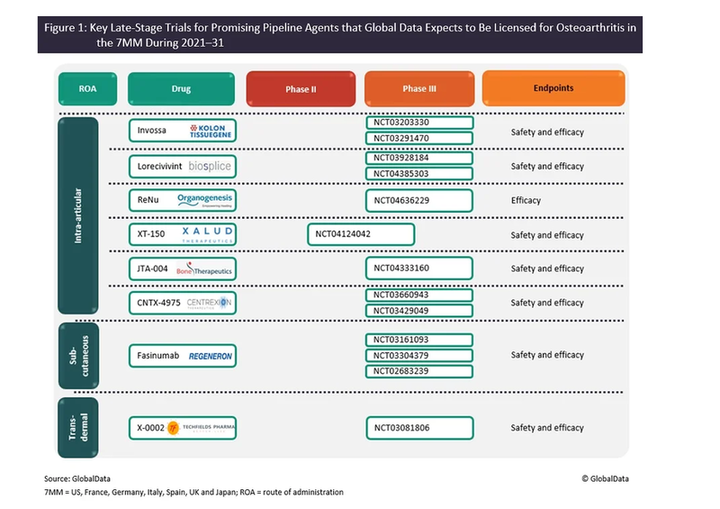
Conventional analgesics offer inadequate pain relief in OA and are associated with a variety of adverse events, thereby driving the development of novel analgesics with improved efficacy and low toxicity. Several analgesics with new mechanisms of action are currently under development, including Regeneron’s fasinumab, Organogenesis Holdings’s ReNu, Bone Therapeutics’s JTA-004, Techfields Pharma’s X-0002, Xalud Therapeutics’s XT-150 and Centrexion Therapeutics’s CNTX-4975. TissueGene’s Invossa, a gene-modified cell therapy, and Biosplice Therapeutics’s lorecivivint, a small molecule inhibitor of the Wnt pathway, are two DMOADs for slowing the progression of joint damage ‒ both are currently in late-stage development.
The limited number of DMOAD products is partially due to the complex pathophysiology of OA and the lack of appropriate outcome measures for progression of joint damage. In addition, the expensive and prolonged clinical trials necessary to determine the disease-modifying effect of new drugs have driven previous developers towards advancing symptomatic treatments that offer immediate therapeutic effects for patients (pain relief) over the development of DMOADs. As a result, the research and development (R&D) strategy is mostly focused on symptomatic treatments, with six pipeline products, rather than DMOAD treatments, which have only two pipeline products.
The figure below shows the key Phase IIb and Phase III therapies currently in the OA pipeline and the companies developing them.
R&D