Company Insight
Sponsored by Alphial
Alphial - Your Trusted Partner for Pharmaceutical Primary Packaging
Alphial designs and produces tubular glass ampoules and vials, delivering precision and flexibility through customer-oriented solutions that respond to the evolving needs of the Pharmaceutical and Cosmetic industries.
Main image: Glass vial sealed with aluminium cap and butyl stopper.
Recently, the company has entered into a strategic agreement with SGD Pharma, resulting in the full acquisition of Alphial. This milestone marks a significant step in Alphial’s journey, combining its expertise in high-performance glass packaging with SGD Pharma’s global reach and resources, creating new opportunities for growth and innovation in the Pharmaceutical industry.
Although officially founded in 2022, Alphial draws on a deep-rooted legacy that dates back to 1968. In 2024, the company expanded significantly through the acquisition of two Italian firms specializing in tubular glass ampoules. Today, Alphial operates across four production sites and employs over 220 skilled professionals.
Our strength lies on an unwavering commitment to product quality, the continuous enhancement of our production flexibility to respond effectively to the evolving needs of the global market, and a well-established presence in over 100 international markets — all while ensuring that every product remains proudly Made in Italy. This positions Alphial as a reliable and globally recognized partner.

Glass ampoules – forming.
We deploy cutting-edge, continuously upgraded inspection systems to ensure every ampoule and vial meets the highest quality standards. These systems form the backbone of our quality assurance process, guaranteeing product consistency and reliability for our clients worldwide.
Our competitive edge is built on the consistency and reliability of our products, reinforced by continuous investments in our production facilities to boost efficiency and support our global market vision. In particular, we have developed recognized expertise in the production of Ready-To-Use (RTU) vials, which we continue to refine and diversify through ongoing research and development, further supported in 2022 by the establishment of a state-of-the-art clean room.
Our mission is clear: to stand by our clients at every stage of the process, ensuring that end users receive safe, effective, and appropriate treatments, delivered through solutions that enable reliable and straightforward administration. At the same time, we are committed to minimizing our environmental footprint through efficient production and waste reduction strategies.
We strongly advocate for the use of glass as a sustainable, fully recyclable material, and actively encourage our clients to make environmentally responsible packaging choices.
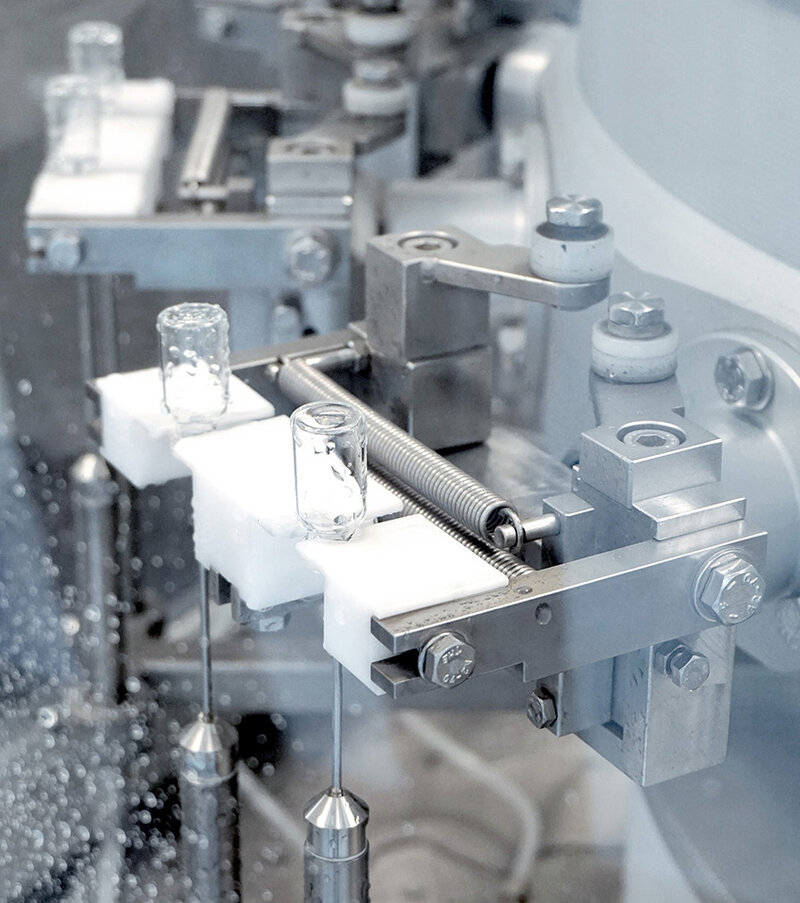
RTU glass vials – LS5 washing process.
At Alphial, our activities are customer-centric, aiming to elevate the service experience while maintaining a firm commitment to sustainability. We carefully select premium raw materials in close collaboration with our suppliers, translating directly into enhanced product performance.
Our production facilities are strategically located across Italy. The sites in Bernareggio (MB), Treviglio (BG), and Perugia (PG) are dedicated to the manufacturing of tubular glass ampoules, while the Felino (PR) plant specializes in tubular glass vials and offers a range of value-added services, including sterilization and depyrogenation of both in-house and third-party containers, through the use of our proprietary LS5® process.
Across our four sites, Alphial operates 55 production lines, with an annual capacity exceeding 1 billion ampoules and 70 million vials. Products are distributed globally through a combination of direct sales, commercial agents, and distributors.
All facilities are ISO 9001 and ISO 15378 certified, and Alphial holds DMFs for China, the United States, and Canada.
Our Felino site features a state-of-the-art cleanroom pharmaceutical department, capable of processing up to 200,000 RTU vials per day under ISO 5 – Class A LAF conditions. Advanced robotic systems reduce human contact, ensuring greater precision, safety, and cost-efficiency.
The cleanroom is equipped with a comprehensive chemical and microbiological laboratory, providing in-depth monitoring and quality control, reinforcing our commitment to excellence in both product quality and environmental safety.
With a solid industrial foundation, a forward-looking mindset, and a strong sense of responsibility toward patients and the environment, Alphial continues to shape the future of glass primary packaging.
Contact information
Alphial S.r.l.
Via Brignano, 81
24047 Treviglio (BG), Italy
Tel.: +39 0521 836471
Email: info@alphial.eu
Web: www.alphial.eu
