Image: Atomwise CEO and co-founder Abraham Heifets
Unmet needs remain in the neuroendocrine tumour treatment paradigm
Through discussions with key opinion leaders, GlobalData has identified the four most important unmet needs in the treatment of neuroendocrine tumours.

Dr Judith M. Sills. Credit: Arriello

Dr Eric Caugant. Credit: Arriello
Neuroendocrine tumours (NETs) have a medium level of unmet need. Most NET patients have slow-growing forms of disease and there are several treatment options that can offer extended overall survival (OS) over multiple years. There is, however, no definitive curative option other than surgery.
Through discussions with key opinion leaders (KOLs), GlobalData has identified the four most important unmet needs in NETs, three of which are clinical and one of which is environmental (Table 1).
One of the two most important clinical unmet needs is predictive biomarkers that can match a patient with the most appropriate therapy. New biomarkers that have recently entered the treatment paradigm, such as tumour mutational burden and NTRK fusions, have helped meet this need but are still rare in the overall paradigm of NETs.
Another important clinical unmet need is safer therapies for patients with low-grade tumours after treatment with somatostatin analogues (SSAs) and more efficacious therapies for patients with high-grade tumours.
At the moment, targeted agents such as Novartis’ Afinitor (everolimus) and Pfizer’s Sutent (sunitinib) are being criticised for being too toxic in low-grade NET patients and for not providing enough clinical benefit when used in high-grade NET patients.
The last clinical unmet need is for well-tolerated agents for somatostatin receptor (SSTR) negative tumours. SSTRs are expressed in the majority of NETs and the few SSTR-negative NET patients have significantly diminished treatment options.
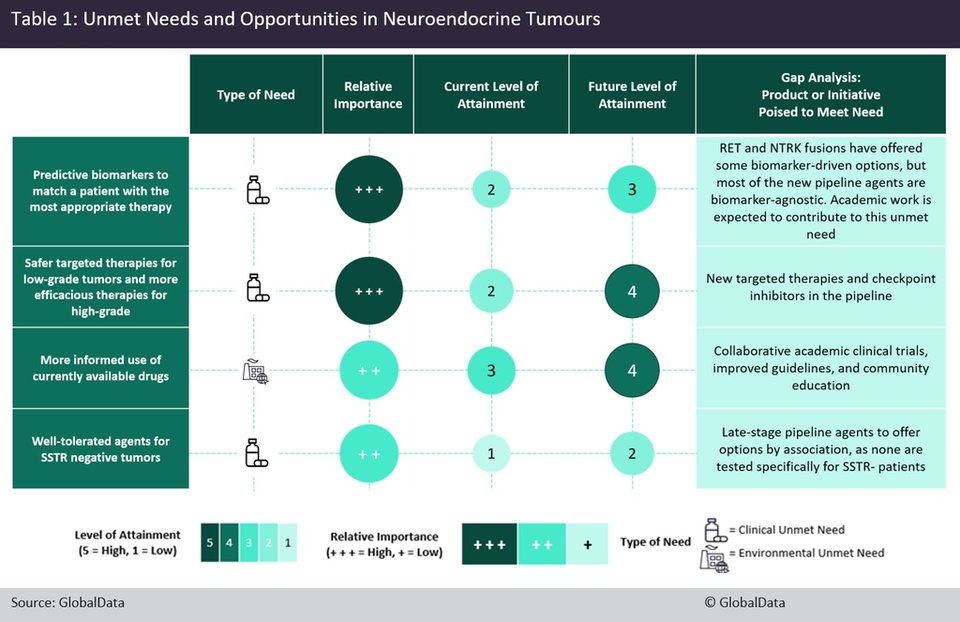
The only environmental unmet need identified by GlobalData is a more informed use of currently available drugs. This is especially pertinent in China, where there is a gap in knowledge about the use of SSAs, but is true of all markets when it comes to the use of the peptide receptor radionuclide therapy, Novartis’ Lutathera (lutetium-177 dotatate).
Given Lutathera’s marketing authorisations in 2017 in the EU and 2018 in the US, several questions about the drug remain unanswered, such as when it can be used in the first line, whether re-treatment after progression on Lutathera is beneficial and whether there is a more appropriate dosing schedule other than the fixed four doses.
While these unmet needs create significant market opportunities, few late-stage pipeline agents are designed to address them. Roche’s Gavreto (pralsetinib) could offer a new biomarker-driven option, but the rarity of RET fusions in NETs may mean that Gavreto will not be effective enough.
Academic work and collaborations are expected to contribute more to new biomarker development and utilisation. Junshi Bio’s Tuoyi (toripalimab) may provide a more efficacious option for high-grade NET patients, but options for SSTR-negative patients are still lacking.
The need for more informed use of current drugs is expected to improve with further collaboration, community education and better guidelines. GlobalData has also identified a need for SSAs that can overcome resistance to octreotide or lanreotide as a key opportunity for drug development.
For pharmaceutical industry data, comment and analysis, visit GlobalData's Pharmaceuticals Intelligence Centre.
Comment from