Image: Atomwise CEO and co-founder Abraham Heifets
Despite benefits, long-acting injectables remain underutilised for schizophrenia
Resistance surrounding the use of long-acting injectables in schizophrenia remains, resulting in lower-than-anticipated uptake, say GlobalData analysts.
Dr Judith M. Sills. Credit: Arriello
Dr Eric Caugant. Credit: Arriello
Discontinuation or non-adherence to antipsychotic treatment is the most common cause of relapse in patients diagnosed with schizophrenia. As such, patients are typically offered continuous antipsychotic pharmacotherapy to prevent recurring episodes of psychosis.
The use of long-acting injectable (LAI) formulations of these antipsychotics can help prevent hospitalisations related to symptom relapse, as these products are administered every fortnight or in one to six-month intervals, versus once or twice-daily oral antipsychotics. This greatly minimises the treatment burden and greatly improves treatment adherence. Despite the benefits of these products, resistance surrounding the use of LAIs in schizophrenia remains, resulting in lower-than-anticipated uptake.
The use of LAI antipsychotics provides an efficient method for monitoring treatment adherence, as they must be administered in a clinical setting; thus they allow for appointment attendance to be tracked. Patients who have a history of good response to oral antipsychotics but adhered poorly to the treatment regimen, resulting in relapse, are eligible to receive LAI therapy. Despite this, some key opinion leaders (KOLs) interviewed by GlobalData argued that LAIs should be prescribed as soon as possible, following first-episode psychosis or during early-stage schizophrenia. As the risk of relapse and poor clinical outcomes increases when maintenance treatment is inadequately managed, KOLs reasoned that the majority of patients could benefit from the improved adherence offered by the LAIs.
Last month, GlobalData surveyed 84 high-prescribing psychiatrists from the seven major markets (7MM: the US, 5EU [France, Germany, Italy, Spain and the UK] and Japan) on current trends in the treatment of schizophrenia, including the prescription of oral versus LAI formulations of one of the most commonly used antipsychotics, aripiprazole, last year (Figure 1).
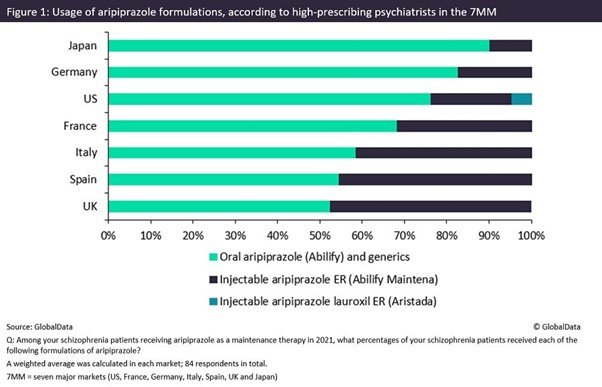
Prescription of daily oral aripiprazole continues to dominate the use of Otsuka and Lundbeck’s once-monthly LAI formulation Abilify Maintena and Alkermes’ Aristada, which can be administered every six weeks. This trend was particularly significant in Japan and Germany with 90.2% and 82.7%, respectively, of patients using oral aripiprazole. There was, however, significant variability across the 7MM, with LAI aripiprazole usage in Spain and the UK reaching 45.4% and 47.5% respectively. Overall, throughout the 7MM, the vast majority (70.2%) of patients receiving aripiprazole last year were prescribed the oral formulation.
There are many reasons for lingering resistance around the use of LAI over oral antipsychotics, with individual preferences from both patients and psychiatrists. For example, there are concerns regarding the long wash-out period of LAI products and the management of the side effects that are common with antipsychotic treatment. In addition, the administration of LAI antipsychotic treatment requires a healthcare professional and does not appeal to patients with needle phobia.
KOLs noted that in an outpatient setting, many psychiatrists are ill-equipped to administer these injectables. This was particularly evident in clinics and practices where they do not have a nurse on staff and the psychiatrist is uncomfortable giving routine injections. This can, unfortunately, act as a barrier to the widespread adoption of LAI as a standard of care for patients with schizophrenia, despite demonstrated benefits to adherence.
With the anticipated patent expiry of Abilify Maintena in the 5EU and US next year and in 2024 respectively, Otsuka and Lundbeck look to launch their next-generation aripiprazole reformulation, an LAI dosed once every two months. GlobalData forecasts that it will launch in the US and 5EU next year. Based on discussions with KOLs, however, the uptake of the aripiprazole two-month LAI is expected to be limited, as the market is crowded with these types of products and, generally, the implementation of an LAI within a standard of care for schizophrenia remains low: on average, less than 30% of aripiprazole prescriptions in the 7MM, according to GlobalData’s research.
Regarding ways to improve the uptake of LAI products, KOLs touted that expansion of the administration possibilities to pharmacies and nurses outside the psychiatric team could improve access to LAI and be particularly beneficial to outpatients. In addition, there is ample opportunity to explore the reasons for the exceptionally diminished use of LAI in Japan and Germany, in comparison with the rest of the 7MM.
MARKET ACCESS