COMPANY INSIGHT
Sponsored by METTLER TOLEDO
United States and European pharmacopeia changes
What Every UV/Vis Lab Should KnowUnited States and European pharmacopeia changes
Pharmaceutical companies are facing the challenge of adapting their workflow to pharmacopeia regulation revisions that became mandatory at the beginning of 2020. Both the United State Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) have dedicated chapters in their respective pharmacopeias on the requirements of UV/Vis spectrophotometers and the verification of their performance.

To ensure customers can comply with the changes, METTLER TOLEDO has adapted its automated performance verification accessories. This editorial provides an overview of the important UV/Vis spectroscopy-relevant changes in both the USP and Ph. Eur., taking a closer look at their impact and the solutions available to ensure compliance.
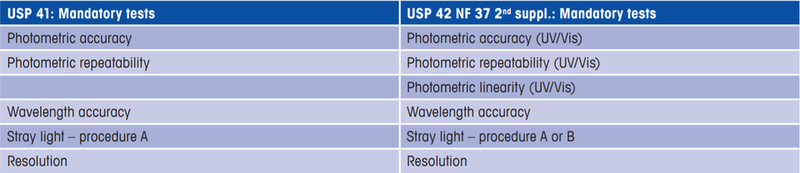
Optical chapter 857 revisions based on the comparison of the 41st to 42nd edition of the United States Pharmacopeia (USP). Changes have been mandatory since January 1st 2020.
Wavelength accuracy
Wavelength accuracy assesses the ability of the instrument to faithfully reproduce wavelengths. The measurement of the wavelength accuracy allows to determine whether shifts of misalignment of the wavelength axis are present. Suitable reference materials for the determination of wavelength accuracy should exhibit narrow and well-defined peaks. USP and Ph. Eur. state different acceptance criteria depending on the reference material used and their wavelength range. For liquid reference materials, the following criteria apply:
USP: ±1 nm below 400 nm; ±2 nm above 400 nm
Ph. Eur.: ±1 nm below 400 nm; ±3 nm above 400 nm
Photometric accuracy
Photometric accuracy is a measure of the ability of the instrument to correctly measure absorbance and to return absorbance values as close as possible to the true value. Photometric accuracy can be determined by measuring solid or liquid filters of known concentration and absorbance values and comparing the measured absorbance with reference absorbance values. A 60 mg/L solution of potassium dichromate (PDC) is used for the ultraviolet range, while a 0.5 A neutral-density (ND) filter covers the visible range. The acceptance criteria are the same for both pharmacopeia and are dependent on the absorbance level of the reference material.
PDC: ±0.010 A below 1.0 A; ±1.0% above 1.0 A
ND: ±0.008 A below 1.0 A; ±0.8% above 1.0 A
For wavelength accuracy and photometric accuracy, according to USP and Ph. Eur., wavelength precision, or repeatability, is not required for array-based spectrophotometers. Thanks to the absence of moving part, array-based instrument are not subject to misalignment in-between measurements.

UV/Vis spectrum of 60 mg/L PDC in 0.001 M HClO4 including the +/- 0.010 A acceptance criteria per wavelength (235, 257, 313, 350 nm).
Photometric repeatability
Photometric repeatability, also referred to as photometric precision by the USP, defines the capacity of a spectrophotometer to return absorbance values over multiple measurements in a reliable and repeatable manner. The same reference materials used to determine photometric accuracy are used. The filters are measured ten times and the standard deviation over all measurements is calculated to assess the repeatability.
The standard deviation ≤ 0.005 A below 1 A; or 0.5% above 1 A, regardless of the used reference material or the wavelength range.
Note: photometric repeatability is not required by the Ph. Eur.
Photometric linearity
Photometric linearity is a measure of the linearity of the measured values (e.g. absorbance) as a function of increasing concentration. This is especially important for quantitative applications. Photometric linearity can be determined with the same procedures and filters used to determine photometric accuracy, however, at three concentrations/different filters covering the range between 0 A and 2 A are used. The USP and the Ph. Eur. state different acceptance criteria for the photometric linearity:
Ph. Eur.: the correlation coefficient R2 must exceed 0.999.
USP: the measured values at all concentration/filters must fulfil the photometric accuracy criteria.
Photometric linearity of a UV7 Excellence spectrophotometer evaluated at the absorption minima (235 (blue) and 313 (grey) nm) and the absorption maxima (257 (orange) and 350 (yellow) nm) of PDC concentrations ranging from 20 mg/L to 220 mg/L.

Stray Light
Stray light is defined as light reaching the detector, which is not part of the analysis. It causes a bias at the corresponding wavelength but does not originate from a contribution of the measured sample. Therefore, the absorbance of the sample is altered by stray light and the sample results include a systematic error. Stray light is determined by measuring a cut-off filter (an aqueous potassium chloride solution) using both the specific wavelength method (SWM) and the solution filer ratio method (SFRM). The acceptance criteria stated by the USP and Ph. Eur. for KCl are as follows:
SWM: Amax ≥ 2.0 A
SFRM: Amax ≥ 0.7 A
Resolution
Resolution defines the resolving power of a UV Vis spectrophotometer and its capability of resolving adjacent structures in a spectrum. Resolution is determined by measuring a solution of Toluene in n-Hexane. The absorption minimum and maximum of a sharp and well-resolved peak are used for the assessment of the resolution. The USP states that the ratio between the minimum and maximum absorbance at respective wavelengths of 267 and 269 nm must exceed 1.3 to provide sufficient resolution, whereas the Ph. Eur. acceptance criteria are stated in the individual monograph. Unlike scanning UV Vis spectrophotometers, array-based instruments do not have a physical slit, which mechanically defines the spectral bandwidth (SBW) of the instrument. The equivalent SBW of an array instrument can be calculated from the resolution of the instrument.
For example, the UV7 Excellence spectrophotometer's resolution is ≥ 1.9, which corresponds to a SBW ≤ 1.0 nm.
For more information about the pharmacopeia changes download the white paper here and how METTLER TOLEDO can help you, please visit here.
Website address: www.mt.com
Telephone number: 1300 659 761
Email address: info.mtaus@mt.com