Image: Atomwise CEO and co-founder Abraham Heifets
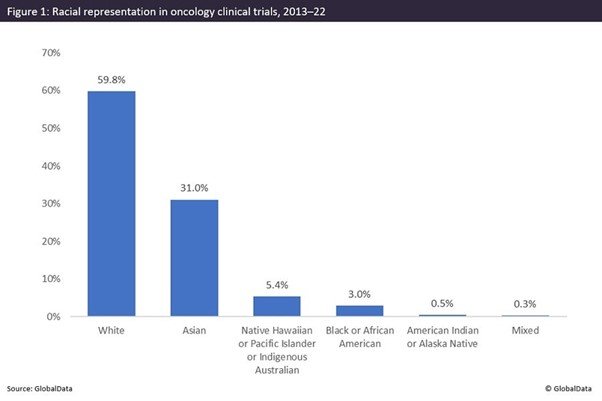
Black participants severely underrepresented in global oncology studies
As per GlobalData, Black participants have had only a 3% involvement in global oncology studies since 2013.
Dr Judith M. Sills. Credit: Arriello
Dr Eric Caugant. Credit: Arriello
The importance of diversity in clinical trials has increasingly become a key issue within the pharmaceutical industry. If trial participants are representative of the wider disease population, clinical trials can effectively assess the safety and effectiveness of new or existing therapeutics. A lack of diversity in clinical trial populations significantly diminishes the quality of data obtained for drug safety and efficacy profiles. This lack of diversity is a significant issue facing the oncology field. GlobalData has found that Black participants are severely underrepresented in oncology studies, with a 3% involvement in global clinical trials from 2013 to the present.
The 3% level of representation serves as a cause for concern owing to data reported by the US National Institutes of Health (NIH), which reveal that, from 2015 to 2019, non-Hispanic Blacks had the highest cancer death rates among all races/ethnicities. This demonstrates that improving Black enrollment in oncology studies is vital so that biological responses to therapies are fully understood, to allow for the administration of the most appropriate treatments. Relative to other therapy areas, oncology has the greatest number of expanded access and companionate use program trials utilising experimental therapeutics. It is therefore essential that diverse race groups can receive innovative therapies and that therapeutics in development are representative of the disease demographic to prevent excess mortality.
A diverse clinical trial population allows researchers to identify discrepancies in responses across population subgroups, ultimately allowing for a better understanding and a more targeted approach in the administration of therapeutics to diverse populations in a real-world setting. Strategies to improve diversity across the pharma industry must be considered where trial populations vastly differ from the disease demographics to ensure that all population subgroups are well-represented in clinical research, especially within the field of oncology.
Clinical Trials