Will the Early Access to Medicines Scheme speed up patient access to innovative drugs?
Of the Early Access to Medicines Scheme approvals given by the UK's Medicines and Healthcare Regulatory Authority (MHRA), almost half are for drugs already authorised in other markets.
The MHRA Early Access to Medicines Scheme (EAMS) is a two-step voluntary process through which companies can apply for a drug to address an unmet medical need, allowing UK patients access to the treatment.
The first step is th MHRA’s designation of a Promising Innovative Medicine (PIM) as an early positive indicator that the drug is intending to treat, diagnose or prevent a life-threatening or seriously debilitating illness.
US biopharma company Global Blood Therapeutics’ drug voxelotor, already approved in the US as Oxbryta, was the first sickle cell drug to receive PIM designation on 14 June. Since the scheme was launched in 2014, however, has there been significant progress in getting innovative drugs to the UK market?
GlobalData’s Pharma Intelligence Centre Drugs database covers drugs that have received PIM designations. These are given to drugs that have completed Phase III trials or, in exceptional circumstances, Phase II trials and flags to investors on drugs’ potential towards getting regulatory approval for the indication of interest.
The second step is the EAMS Scientific Opinion assessment, which is only issued once a drug meets the EAMS safety criteria and with a positive benefit, namely risk ratio. Only then can the National Health Service (NHS) commission the free supply of the drug from the company for patients.
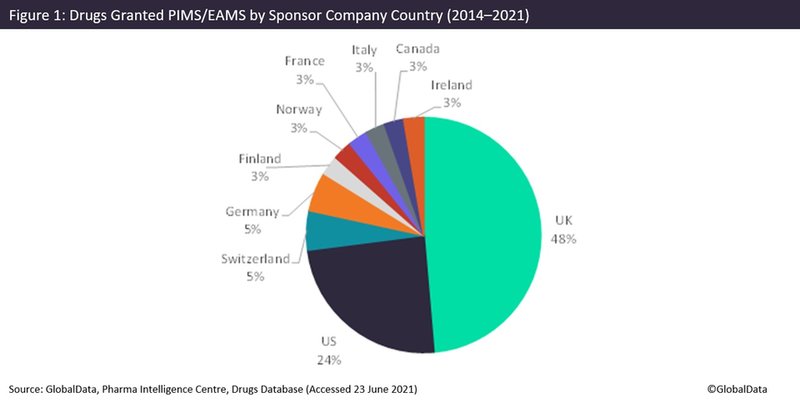
The UK has almost half of its pharmaceutical companies receiving PIM/EAMS status, at 48%, with the US receiving 24% and the remaining companies predominately coming from Europe, according to GlobalData’s Pharma Intelligence Centre Drugs database (Figure 1).
Out of the total number of companies that have received PIM/EAMS status, 46% are for drugs that have already received marketing authorisation in other markets. This begs the question, therefore, of how much progress this scheme will contribute to getting novel drugs to market.
For example, Pfizer received PIM designation last October for abrocitinib in Phase III in the UK for the treatment of severe atopic dermatitis. The drug was then issued a positive EAMS scientific opinion in February.
Sanofi also received PIM designation last October for avalglucosidase alfa in Phase III in the UK for Pompe disease, and the drug was issued a positive EAMS scientific opinion in March.
According to a survey of biopharma companies interviewed in GlobalData’s Brexit and the Healthcare Industry report on 27 April, 66% of the companies interviewed indicated that the regulatory implications of the UK leaving the European Economic Area EEA remained the highest factor impacting healthcare after Brexit.
With the UK no longer being part of the EU’s regulatory system, the additional burden of clinical trials and impact on the market authorisation of drugs will need to be closely watched.
For pharmaceutical industry data, comment and analysis, visit GlobalData's Pharmaceuticals Intelligence Centre.
Market Insight from